Abstract
Introduction: Mass cytometry (MC) is a next generation flow cytometry (FC) platform that enables high-dimensional functional and phenotypic analysis of single cells. The technique uses mass spectrometry to detect metal-conjugated antibodies on single cells to evaluate intra- and extra-cellular targets. A key advantage of MC over standard fluorescence-based FC is that compensation for spectral overlap is not required due to the use of heavy metal ion tags in place of fluorescent tags. This allows simultaneous measurement of ≥ 40 different parameters per single cell in comparison to the 3-5 parameters that comprise a typical FC panel for platelet analysis. This is the first study to demonstrate the power of MC for the detailed analysis of platelet surface markers in healthy subjects and in patients with inherited platelet disorders.
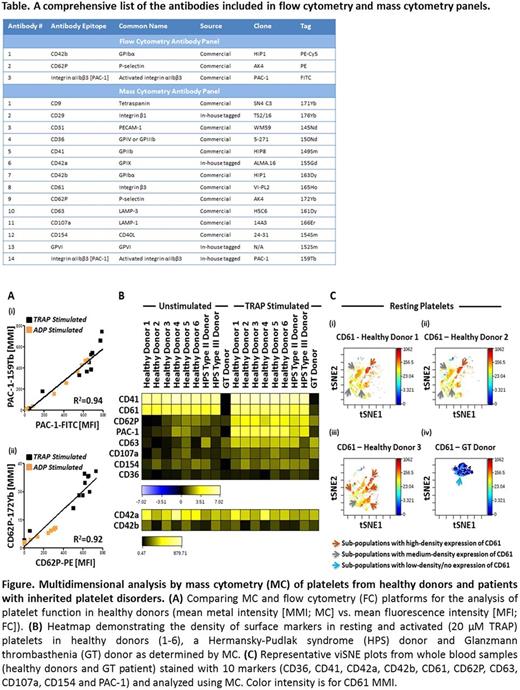
Methods: We compared MC and FC in the analysis of healthy donor platelets and patients with Hermansky-Pudlak syndrome (HPS) Type II/III and Glanzmann thrombasthenia (GT). IRB-approved informed consent was obtained prior to blood collection. Citrate anticoagulated whole blood was stained with commercially sourced fluorescently-labeled antibodies and, in parallel, with a single MC cocktail containing 14 metal-tagged antibodies directed towards an array of platelet surface markers. The MC antibody panel comprised a collection of commercially available and custom in-house metal-tagged antibodies (Table). Samples were stained in the presence or absence of thrombin receptor activating peptide (TRAP) or adenosine diphosphate (ADP), fixed and analyzed using a Helios Mass Cytometer or BD FACSCalibur flow cytometer. Data were analyzed using FlowJo/CytoBank and presented as mean metal intensity (MMI; MC) or mean fluorescence intensity (MFI; FC).
Results: To compare the use of MC and FC for analysis of platelet function, TRAP and ADP dose-response curves were constructed using PAC-1-FITC/159Tb and CD62P-PE/172Yb binding as functional readouts of healthy donor platelet activation. The sigmoidal curves obtained using both platforms were comparable, and results highly correlated (R2 ≥ 0.9; Figure A). MC enabled simultaneous quantitation of additional platelet surface markers (CD9, CD29, CD31, CD36, CD41, CD42a, CD42b, CD61, CD63, CD107a, CD154 and GPVI). As measured by MC, ADP and TRAP stimulation increased the platelet membrane surface expression of CD9, CD41, CD61, CD63, CD107a, CD154 and GPVI and decreased CD42a and CD42b. Surface expression of CD29, CD31 and CD36 as determined by MC remained constant following stimulation. HPS platelets demonstrated reduced PAC-1 binding and normal P-selectin (CD62P) surface expression following TRAP stimulation as compared to healthy donor platelets (Figure B). Interestingly, the type II HPS patient had a significantly elevated platelet surface level of CD63 compared to the healthy donor and HPS Type III donor; a feature that may differentiate the two forms of HPS. GT platelets, as expected, lacked surface CD41 and CD61 and this corresponded with a lack of PAC-1 binding following TRAP stimulation. MC revealed the surface level expression of CD29, CD154 and GPVI to be reduced, CD36 to be significantly elevated, and CD42a, CD63 and CD107a to be similar on GT platelets compared to healthy donor platelets. To take advantage of the high number of parameters simultaneously measured on each cell using MC, results were analyzed using visual stochastic neighbor embedding (viSNE), a tool to generate an optimized 2-dimensional representation of high-dimension data. This analysis revealed platelet sub-populations which were common between healthy subjects (Figure C, healthy donors 1, 2), as well as sub-populations that were unique to particular donors (Figure C, healthy donor 3 vs. 1, 2).
Conclusions: Simultaneous analysis of multiple platelet surface markers by MC reveals previously unrecognized platelet sub-populations in healthy donors and provides unique insights into the platelet populations that exist in patients with inherited platelet disorders. MC can be used as a more powerful and efficient tool than FC to assess the heterogeneity of platelets across donors and to characterize platelet populations that exist in patients with inherited platelet disorders. Rigorous identification of these platelet sub-populations may enable future determination of their role in health and their possible utility as biomarkers in disease.
Michelson: Pfizer: Research Funding; Eisai: Research Funding; Sysmex: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Instrumentation Laboratory: Consultancy; Baxalta: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; GLSynthesis: Research Funding; Ionis: Research Funding; Ironwood: Research Funding; Elsevier: Patents & Royalties. Frelinger: Baxalta: Research Funding; GLSynthesis: Research Funding; Ironwood: Research Funding; Ionis: Research Funding; Pfizer: Research Funding; Sysmex: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.